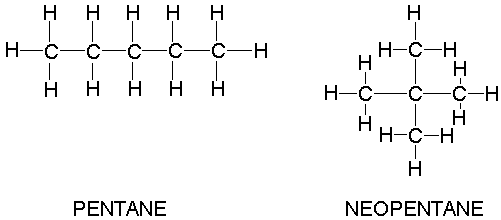
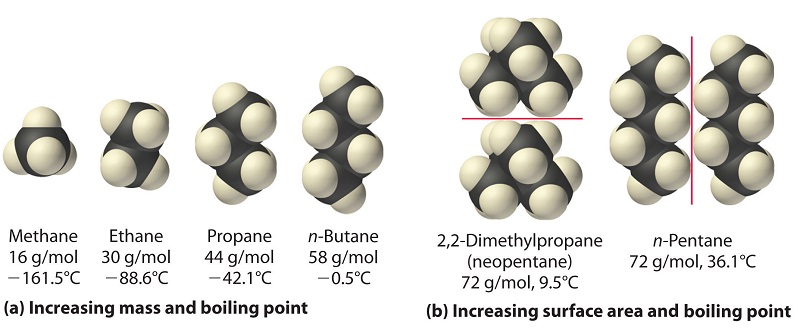
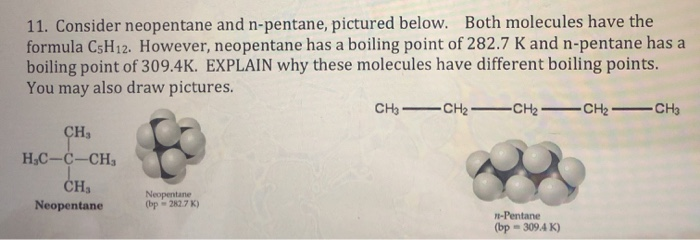
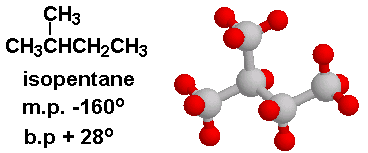
organic chemistry - Why does neopentane have a higher melting point than n- pentane? - Chemistry Stack Exchange

Arrange the following compounds in the descending order of their boiling pointsa) n - pentaneb) isopentanec) neopentane
Why Isomers of a compound have different Boiling point (like Isomers of pentane) why force of attraction is not involve in it? - Quora

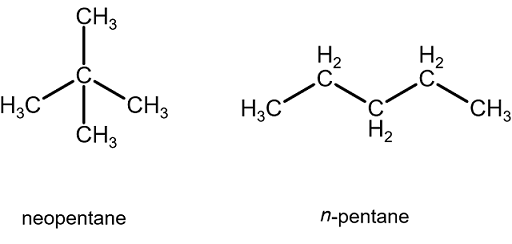
why neopentane has higher melting point than n pentane - Chemistry - Chemical Bonding and Molecular Structure - 13416933 | Meritnation.com

Arrange the following compounds in the descending order of their boiling pointsa) n - pentaneb) isopentanec) neopentane
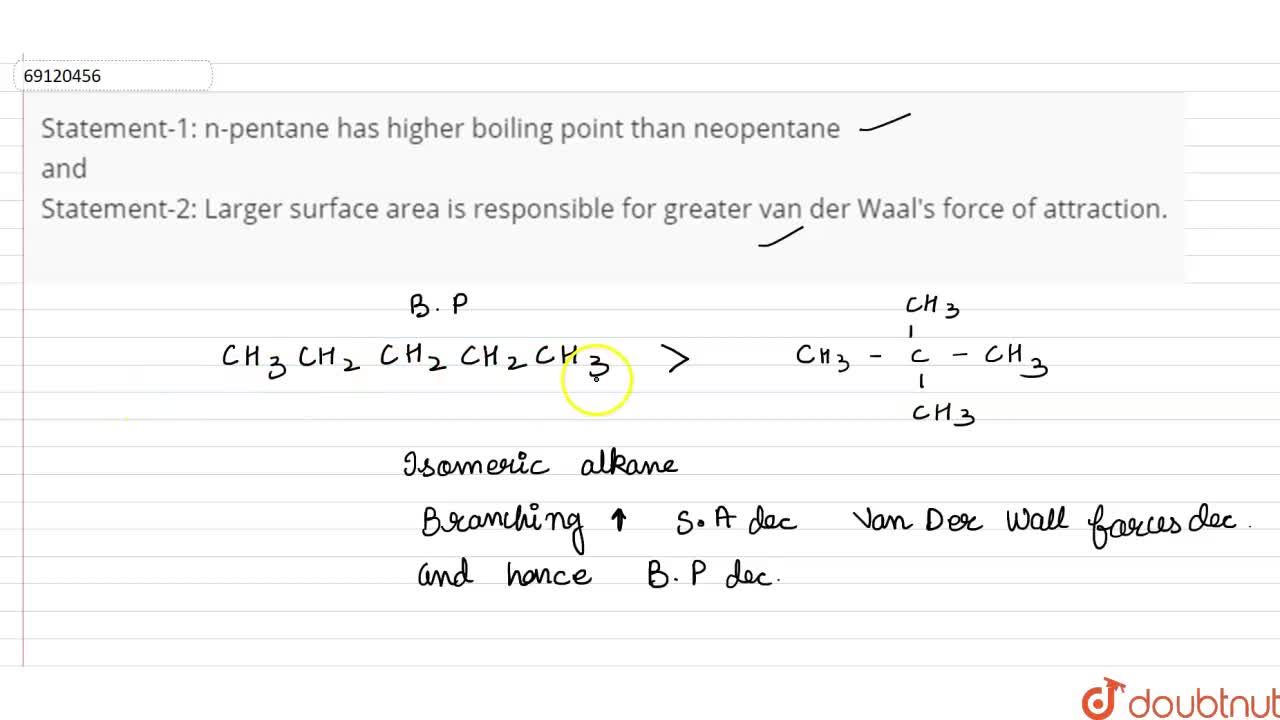
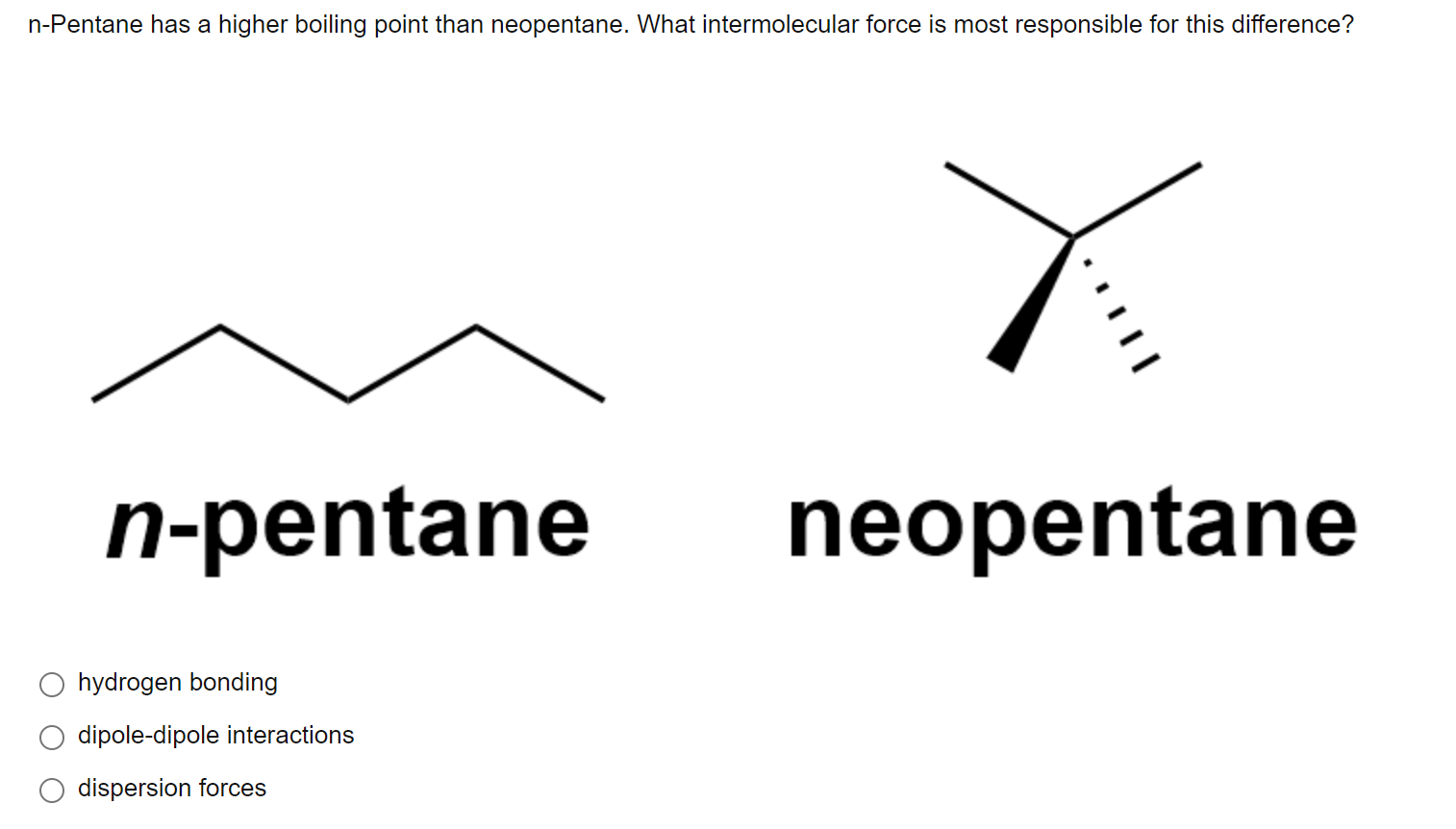
Statement-1: n-pentane has higher boiling point than neopentane and Statement-2: Larger surface area is responsible for greater van der Waal's force of attraction.

organic chemistry - Why does neopentane have a higher melting point than n- pentane? - Chemistry Stack Exchange

Rank these compounds from highest to lowest boiling point. a. pentane b. neopentane c. isopentane | Homework.Study.com
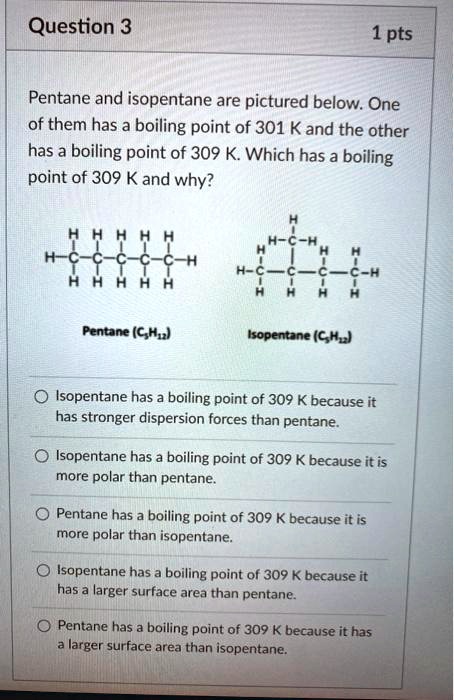
SOLVED: Question 3 1 pts Pentane and isopentane are pictured below. One of them has a boiling point of 301 K and the other has a boiling point of 309 K Which