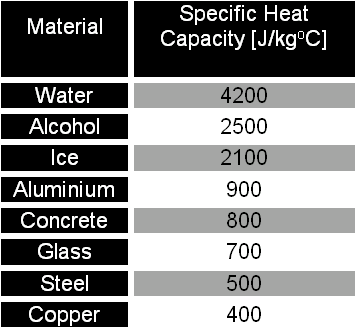
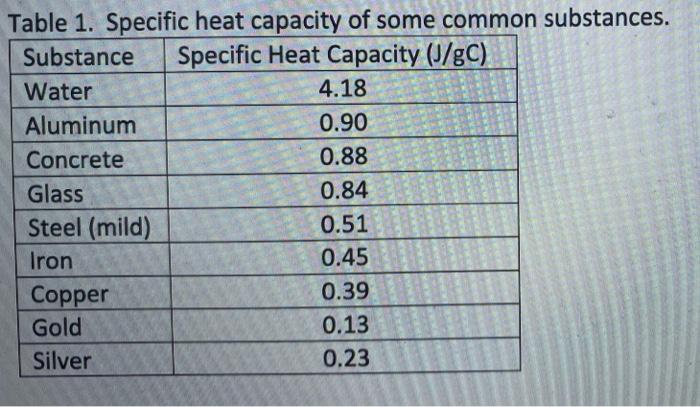
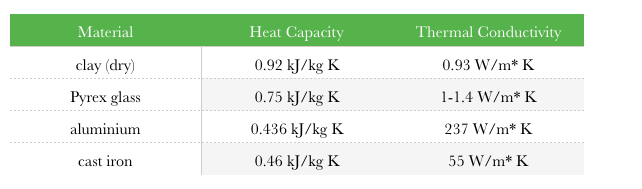
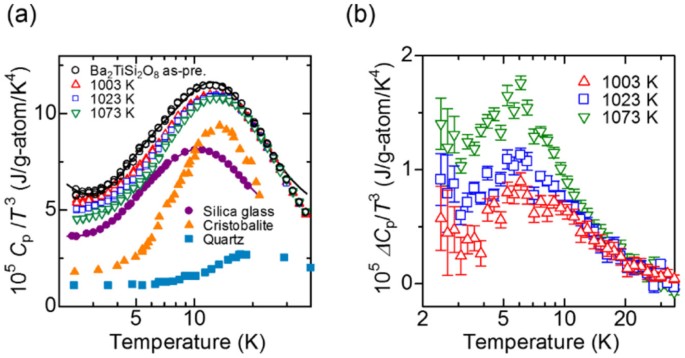
Specific heat and magnetization studies of spin-glass like transition in nanogranular Cu90Co10 ribbon - ScienceDirect

Specific heat capacities for an as-deposited thin toluene film (solid... | Download Scientific Diagram
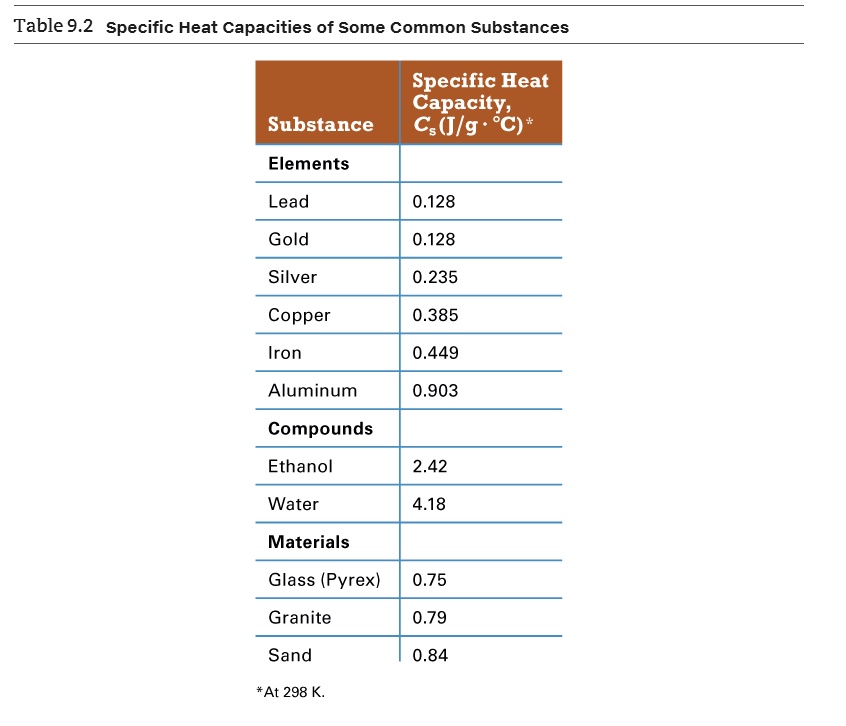
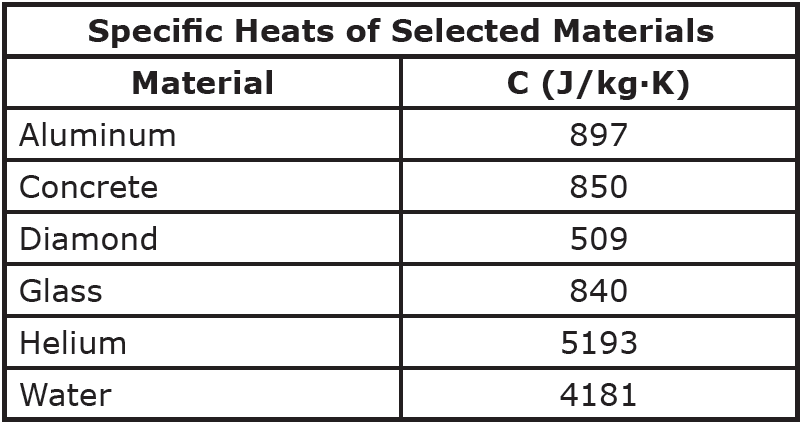
SOLVED: Table 9.2 Specific Heat Capacities of Some Common Substances Specific Heat Capacity; Cs(Jlg. *C) Substance Elements Lead 0.128 Gold 0.128 Silver 0.235 Copper 0.385 Iron 0.449 Aluminum 0.903 Compounds Ethanol 2.42

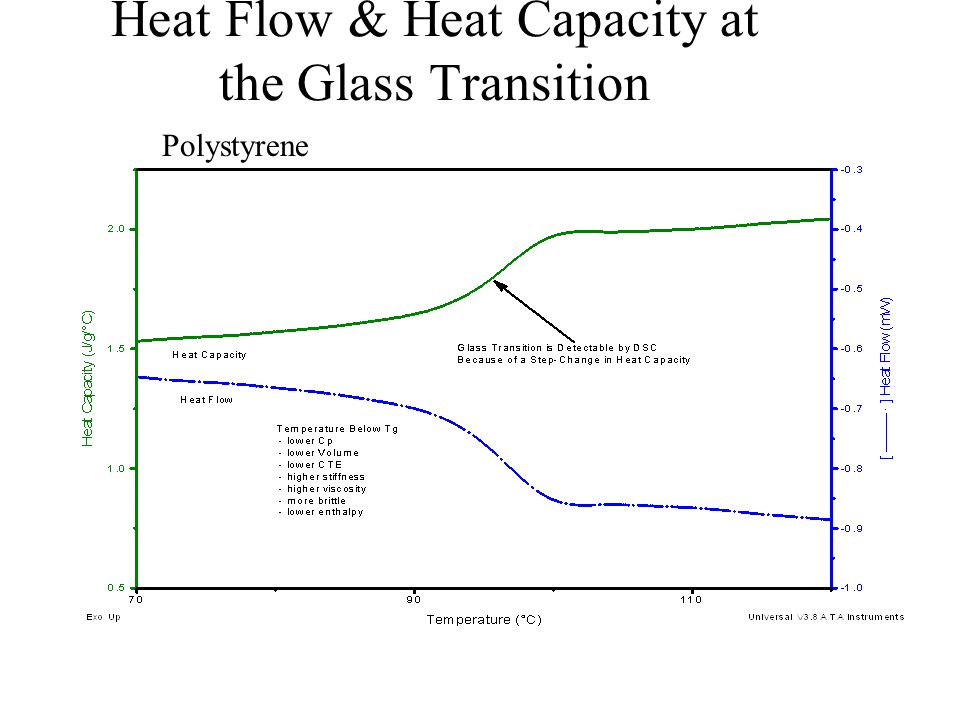
Heat capacity measurements and modeling of polystyrene glass transition in a wide range of cooling rates - ScienceDirect

Unveiling the Dependence of Glass Transitions on Mixing Thermodynamics in Miscible Systems | Scientific Reports
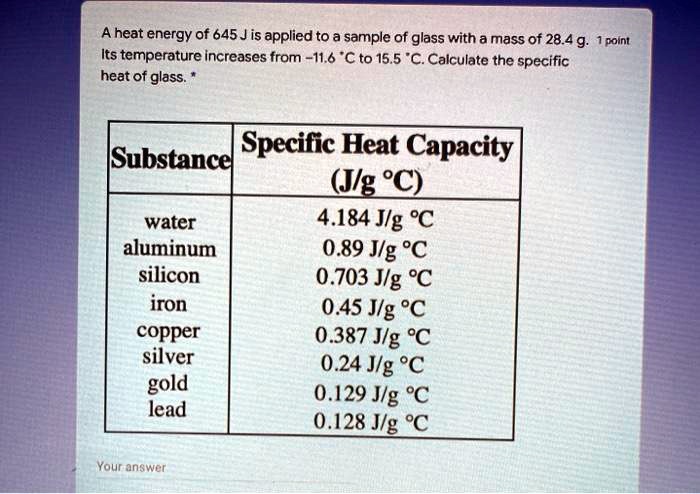




![PDF] Heat capacity at the glass transition | Semantic Scholar PDF] Heat capacity at the glass transition | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/ee44b16b7fd93929389afe67bfaf1a6bd994e5b3/5-TableI-1.png)






.jpg)